
Severe Injuries And Medical Conditions From Recalled Phillips® Brand CPAP & BiPAP Devices
Victims of severe injuries and medical conditions from recalled Phillips® brand CPAP & BIPAP devices can finally seek justice and compensation.
Those eligible must have used a Phillips CPAP, or BiPAP RECALLED device for 4 Years or longer before Thursday, February 25, 2021 [09-25-2021], did not smoke while using the device, and were diagnosed with cancer of the:
- Lungs;
- Mouth;
- Nose; or,
- Larynx;
Or were diagnosed with lung injuries such as;
- Chronic asthma;
- Pneumonitis;
- COPD;
- Pulmonary fibrosis; and,
Have your device number or registration details for receiving a replacement device.
Important consumer information on recalled CPAP or BiPap Devices
Consumers had to register their recalled device to be eligible to receive a replacement device. Those who registered their recalled device received a registration number provided by Philips. Once registered Philips would have placed you on a priority list and you may now have received the replacement device.
If you are need to registering for a replacement deviceTo register for a replacement device, consumers have to enter their name and device Serial Number (S/N) (located on the back of the Philips recalled device), into the following Philips website: https://www.philipssrcupdate.expertinquiry.com/?ulang=en If the Serial Number is not on the recall list, it will not allow the client to complete registration and replacement process.
What if I can’t find the registration number and/or serial number?
Consumers can call the Philips helpline set up for this purpose: (877) 907-7508.
Clients who call the above number will be asked to verify:
- Last name
- Zip Code
- Last (4) digits of the phone number they used to register their device.
Important Update
The FDA just dropped an update on that Philips sleep apnea machine recall, and it's quite an eye-opener. They're saying these machines might be connected to a whopping 561 reported deaths.
Since April 2021, the FDA has been flooded with over 116,000 reports about these respiratory devices. They've been breaking down and causing some serious health risks. This includes talking, choking, inhaling weird particles, and even an increased chance of cancer.
These devices, used for sleep apnea and similar disorders, were made with a foam called polyester-based polyurethane (PE-PUR). However, this foam breaks down over time, and the little bits can end up in the airways of the people using them. The FDA warns you might end up breathing in these "black pieces of foam" or some invisible chemicals.
After the first recall of over 5 million devices in 2021, Philips tried to sort out the issue. But they still fail to meet safety standards or consumer expectations.
Philips has now agreed to stop selling these devices in the U.S., primarily because of the filing of a proposed class action settlement.
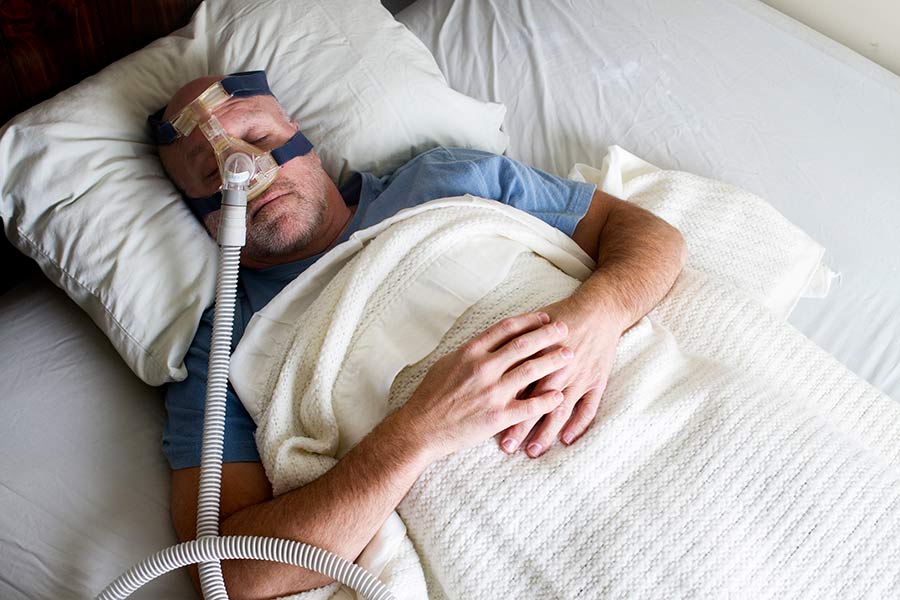
Health Dangers From Using a Recalled Phillips CPAP or BiPAP
Phillips® CPAP and ventilation machines are causing damage—including personal injuries and cancer diagnoses—that is being reported nationwide.
Free Case Review
*Consent: By clicking the "NEXT" or "SUBMIT" button, I consent to receive recurring auto dialed and/or text messages from USA Assistance Team, and Tort Intake Professionals LLC or our partners in order to discuss my legal options and at the telephone number I provided. I also consent to the USA Assistance Team Privacy Policy and Terms of Use. I understand that I may receive a call even if my telephone number is listed on a Do Not Call list and that my consent is not a requirement of purchase. I understand that my telephone company may impose charges on me for these contacts and that I can revoke this consent at any time. For SMS campaigns: Text STOP to cancel and HELP for help. Msg & data rates may apply. Recurring msgs up to 27 msgs per month.
Consumers with damage may qualify for a case evaluation
We offer a free, private case review for victims of recalled Phillips® brand CPAP & BIPAP devices who aren’t satisfied with the company's response.
100% Free & Secure Case Evaluations
Phillips® brand CPAP & BIPAP Device Damage Info To Prepare Consumers
Why Did Phillips® Respironics Recall These Breathing Machines?
The foam that the company embedded in millions of ventilators and sleep apnea machines to reduce noise was found to break down, sometimes because of heat and humidity.
The potential risks of particulate exposure if inhaling or swallowing pieces of PE-PUR foam include:
Irritation to the skin, eyes, nose, and respiratory tract (airway)
Inflammatory response
Headache
Asthma
Toxic or cancer-causing effects on organs, such as kidneys and liver
The potential risks of inhaling chemicals released into the device's air tubes from the PE-PUR foam include:
Headache
Dizziness
Nausea or vomiting
Hypersensitivity reaction, such as an allergic reaction
Toxic and cancer-causing effects
Compensation Begins With A Free Online Case Review
Victims of Phillips® brand CPAP & BIPAP devices deserve to get the compensation and justice they deserve from big companies that resist delivering proper payouts.
Phillips® Brand CPAP & BIPAP Device Damage FAQ: Questions the Health Risks
What Does the Government Say About The Health Risks?
The FDA has reported that when the foam breaks down, the material can move through the device and be inhaled or ingested. The agency said users could experience headaches, asthma, inflammatory conditions, respiratory tract problems, and “toxic or cancer-causing effects to organs,” among other health complications.
The FDA has advised consumers with recalled machines to consult their doctors about the best course of action, noting that some users may face more significant health risks if they stop using them altogether.
What Does Phillips® Say About the Health Risks?
When the recall was announced, Phillips® reported that the defect could cause short- and long-term health risks, including life-threatening conditions. Previously, two internal health hazard evaluations launched by Phillips® had concluded that the risk to people who used the machines was “unacceptable.”
In recent months, Phillips® has reported that initial testing was limited and based on a “worst-case scenario” and that new tests on the DreamStation® and similar devices have found the foam breakdown is “unlikely to result in an appreciable harm to health in patients.”
How Can I Register for a Replacement CPAP Machine or Other Device?
Customers whose machines were recalled are supposed to be able to get replacements from Phillips®, which has a patient registration site.
Though Phillips® has said millions of replacement devices have been sent out, customers have reported the process is riddled with delays.
To register, Phillips® requires the recalled device's name and serial number. The company also requires a current doctor’s prescription.
Discover why our clients get their cases settled and damages paid fast.
Our attorneys have a track record of getting justice and deserved compensation.
Please seek the advice of a medical professional before making health care decisions. This advertisement is not associated with a firefighting foam manufacturer or any government agency.
www.usaassistanceteam.com is the property of Shield Legal LLC. 7180 Pollock Drive, 2nd Floor, Las Vegas, NV 89119
This website is not part of the Facebook website or Facebook, Inc. Additionally, this site is NOT endorsed by Facebook in any way. FACEBOOK is a trademark of FACEBOOK, INC.
ATTORNEY ADVERTISING. This Website is not intended to provide medical advice. Consult your doctor or physician before starting or stopping any medication.
Discontinuing a prescribed medication without your doctor’s advice can result in injury or death. are not an indication of future results. Every case is evaluated on its own facts and circumstances. Valuation depends on facts, injuries, jurisdiction, venue, witnesses, parties, and testimony, among other factors. No representation is made that the quality of legal services to be performed is greater than the quality of legal services performed by other lawyers. USA Assistance Team does not itself provide legal services. Cases will be referred to third party attorneys and law firms. Do not rely on this advertisement in making any medical decision. Please call your physician before making any medical decision, including altering your use of any drug. Court costs and case expenses may be the responsibility of the client. Not available in all states. This advertisement is not intended as a testimonial, endorsement or dramatization, and does not constitute a guarantee, warranty, or prediction regarding the outcome of your legal matter, either expressed or implied. Anyone considering a lawyer should independently investigate the lawyers' credentials and ability, and not rely upon advertisements or self-proclaimed expertise. Only persons age 18 or older have permission to access our Service. Our Service does not address anyone under the age of 13("Children").
Privacy Policy | Terms and Conditions | CCPA Privacy Notice | Do Not Sell My Info
©2024 USA Assistance Team. All Rights Reserved